The Neutral Point of the Ph Scale Is
A pH of 7 is neutral. High on the pH scale.

Acidic Basic Neutral Solutions Determining Ph Video Lesson Transcript Study Com
For recording purposes the numbers to the right of the decimal point in the pH value are the significant figures.
. This value is considered neutralneither acidic or basic. Anything above 7 is considered to be basic. Explain the dynamics of the carbonic acidbicarbonate buffer system as a function of the changes in acidity and alkalinity in.
More alkaline solutions have higher pH greater than 7. Acidic solutions have pH values less than 7. Derive the scale of pH from the property ionization of water.
That is the neutral point on the pH scale at this higher temperatureA solution with a pH of 7 at this temperature is slightly alkaline because its pH is a bit higher than the neutral value of 614. Table 1 has examples of substances with different pH values Decelles 2002. It tells how acidic or alkaline a substance is.
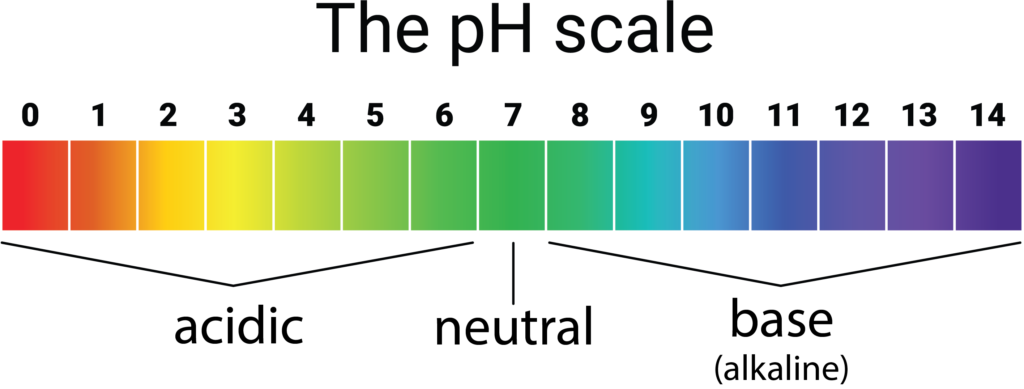
Acidic substances have pH values ranging from 1 to 7 1 being the most acidic point on the pH scale and alkaline or basic substances have pH values ranging from 7 to 14. Thats why 7 is neutral. YOU MIGHT ALSO LIKE.
PH values lower than 7 are acidic and pH values higher than 7 are alkaline basic. PH or potential of hydrogen is a scale of acidity from 0 to 14. Indicate the neutral point and the acidic and basic areas 3.
Buffers are important in biological systems because of their ability to maintain constant pH conditions. The pH Scale. PH is really a shorthand for the concentration of hydronium ions in solution or solvent-H protonated cationsThe point of pH 7 being neutral is dependent on the fact that the usual medium is water.
Indicator A substance that changes color in the presence of an acid or base. Since 10 10 -7 has two significant figures the pH can be reported as 700. The pH scale uses a range from 0 to 14 with 70 indicating neutrality.
Neutral in water is pH 7 is only neutral because there is an equal concentration of hydroxide ions. Values on the scale range from 0-14. Concentration of Hydrogen ions goes down.
Concentration of Hydrogen ions goes up. This scale is logarithmic so there is a 10-fold difference in strength between each number. Cabbage Juice litmus paper The pH Scale.
The pH scale ranges from 0 to 14 with a pH of 7 being neutral. Normal clean rain has a pH value of between 50 and 55 which is slightly acidic. A logarithmic scale condenses the range of acidity to numbers that are easy to use.
The pH scale ranges from 0 to 14 and indicates how acidic or basic a substance is. Explain the dynamics of the carbonic acidbicarbonate buffer system as a function of the changes in acidity and alkalinity in. PH of Acids and Bases.
Pure water is neutral neither an acid nor base. What happens when the pH goes up. Neutral solutions are exactly pH 7.
Hydrogen ions The neutral point on the pH scale. Numbers beginning at 70 and moving toward 0 indicate acidity while the numbers beginning at 70 and moving toward 14 indicate alkalinity so the scale divides acids from bases. But mix an acid with water and the water molecules will act as bases.
PH -log 10 H where H is the concentration of H expressed in molesliter. The pH Scale A scale developed in order to determine how acidic or basic a substance is. As such it sits smack in the middle of the pH scale at 7.
There are bases secreted in our intestines that neutralize the acid from our stomach. 15 rows It means that the pH is determined on basis of the hydrogen ions present in the solution. Pure water has a neutral pH of 7.
Consider a solution with H 10 10 -4 M. 7 Water What happens when the pH goes down. Low on the pH scale.
Explain how hydrophobic interactions form. Anything below 7 is considered to be acidic. For those who want a more complicated answer pH is defined.
Substances which are not acidic or alkaline neutral usually have a pH of 7 this is the answer to your question. More acidic solutions have lower pH less than 7. Each whole pH value below 7 the neutral point is ten times more acidic than the next higher value.
A pH greater than 7 is basic. Solutions having a value of pH ranging 0 to 7 on pH scale are termed as acidic and for the value of pH ranging 7 to 14 on pH scale are known as basic solutions. The scale has values ranging from zero the most acidic to 14 the most basic.
At 100C the pH of pure water is 614. Because it is a logarithmic scale each number is 10 times more powerful or less powerful than the next number. What is a buffer.
The pH scale goes from 1 to 14 with 7 being neutral. The possible values on the pH scale range from 0 to 14. What is a buffer.
Most images show the pH scale going from zero to 14. The pH of a solution varies from 0 to 14. In water the concentration of protons H or rather hydronium ions H3O.
A pH less than 7 is acidic. The pH of pure water or any neutral solution is thus 700. Buffers are solutions that moderate pH changes when an acid or base is added to the buffer system.
The lower a substances pH the more acidic it is. As you can see from the pH scale above pure water has a pH value of 7. The pH scale is a measurement system used by chemists to indicate the concentration of this substance in a solution.
What is the neutral point on the pH scale. Derive the scale of pH from the property ionization of water. At 100C a pH value of 614 is the new neutral point on the pH scale at this higher temperature.
The pH scale is used to classify solutions as acidic alkaline or neutral. Explain how hydrophobic interactions form. A pH of 8 is 10 times more basic than water and a pH of 9 is 100 times more basic and so on.
The amount of H that is made in pure water is about equal to a pH of 7. Indicate the neutral point and the acidic and basic areas 3.

Ph Scale U S Geological Survey

What Does It Mean To Have A Neutral Ph Aseptic Health
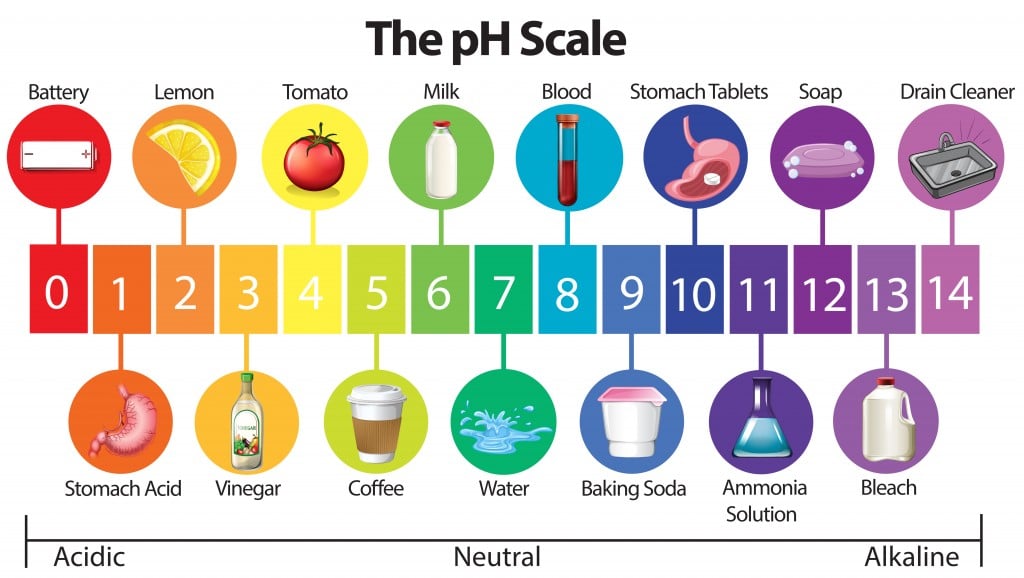
Why Does The Ph Scale Range From 0 To 14 Can It Go Beyond That Range
Comments
Post a Comment